Photothermal CO2 Capture Systems
The efficiency of current liquid-amine carbon capture methods is limited by the energetically expensive step whereby CO2 is separated from capture solutions. This step requires large energy inputs to heat a solution to release CO2 and regenerate solvent. Previous work has shown that the photothermal heating of nanoparticles initiates CO2 release without the typical energy inputs required to heat the bulk solution. This photothermal phenomenon has the potential to lower the overall energy cost of current carbon capture methods by increasing the efficiency of CO2 release using localized photothermal heating and by using solar energy instead of coal, gas, or biomass.
We are utilizing this photothermal phenomenon to develop a system which can passively pull CO2 out of the atmosphere and continuously regenerate solvent via solar energy. We have demonstrated this system at bench-scale by suspending carbon-black nanoparticles in capture solution and implementing it in a passive-flow system using a semipermeable membrane. The efficiency of this system is largely dependent on the composition and thickness of the membranes, and the surface area to volume ratio (A/V) of the solvent-air interface.

Thermal stripping of CO2 from aqueous solvents occurs at high temperatures which results in evaporative losses and thermal degradation of amines. Regeneration of MEA is energy intensive, using 80-85% of operating costs.
Photo-thermal initiation of carbon black nanoparticles (NCBs) increases regeneration efficiency of capture solutions1 and reduces energy costs.
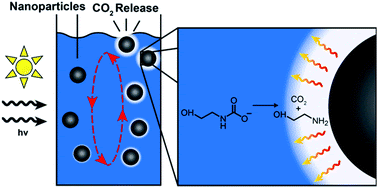

The primary goal of this system is to passively draw CO2 out of the atmosphere using solar light as the energy source. This design allows for a more portable and robust alternative to current liquid-amine capture systems.


